Authors
Lindsay A.L. Bazydlo
Department of Pathology
University of Virginia
Charlottesville, VA, United States
Maximo J. Marin
Department of Pathology Immunology and Laboratory Medicine
Division of Infectious Diseases
University of Florida
Gainesville, FL, United States
Anna E. Merrill
Department of Pathology
University of Iowa
Iowa City, IA, United States
Louise M. Man
Department of Hematology/Oncology
University of Virginia
Charlottesville, VA, United States
Olajumoke O. Oladipo
Department of Pathology and Laboratory Medicine
Penn State Milton S. Hershey Medical Center
Hershey, PA, United States
Neil S. Harris
Department of Pathology Immunology and Laboratory Medicine
Division of Infectious Diseases
University of Florida
Gainesville, FL, United States
Previous presentation: This work was presented on July 28, 2025, at ADLM 2025, Chicago, IL.
This document was approved by the Academy Content Development Committee and Academy Council in June 2025 and the ADLM Board of Directors in July 2025.
https://doi.org/10.1093/jalm/jfaf155
© Association for Diagnostics & Laboratory Medicine 2025. All rights reserved. For commercial re-use, please contact [email protected] for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact [email protected].
Background: Coagulation testing has an important role in the diagnosis, monitoring, and therapeutic decision-making for patients with abnormal hemostasis. As the field of anticoagulation options has expanded, the introduction of direct oral anticoagulant (DOAC) drugs has provided an option for patients utilizing drugs that do not require routine monitoring. Patients on these drugs may still need coagulation testing for prognostic purposes, and there are a number of nuances to consider when performing coagulation testing in patients who are prescribed DOACs.
Content: This document provides guidance on the tests that may or may not be impacted by the presence of DOACs in the blood. A discussion is provided about specific coagulation tests used and the impact of testing samples with a DOAC present. Options are presented to help mitigate the impact of DOACs on the testing. In addition, this document discusses how to test for and interpret DOAC concentrations in specific patient populations.
INTRODUCTION
The introduction of direct-acting oral anticoagulants (DOACs) has been a major advancement in the management of anticoagulation. Previously, outpatient oral anticoagulation was limited to vitamin K antagonists, namely warfarin. Vitamin K agonists have numerous drug-drug and dietary interactions. They also require frequent laboratory monitoring for therapeutic ranges and require dosing changes chronically. In contrast, DOACs allow for oral anticoagulation with fixed doses that have relatively predictable pharmacokinetic characteristics and do not generally require routine therapeutic monitoring. These drugs also have fewer drug–drug and drug–food interactions. In a relatively healthy patient population, the duration and efficacy of the DOACs are generally dependent on half-life, age, and renal excretion or clearance. Table 1 summarizes indications, mechanisms of action, and the pharmacokinetic properties of the DOACs (1, 2). Commercially available DOACs in the United States currently include dabigatran (Pradaxa®), rivaroxaban (Xarelto®), apixaban (Eliquis®), and edoxaban (Savaysa®). Dabigatran is a competitive direct thrombin inhibitor, while rivaroxaban, apixaban, and edoxaban are competitive direct factor Xa (FXa) inhibitors.
|
Anticoagulant |
|||||
|
Anticoagulant characteristics |
Dabigatran (Pradaxa®) |
Rivaroxaban (Xarelto®) |
Apixaban (Eliquis®) |
Edoxaban (Savaysa®) |
|
|
FDA-approved uses |
NVAF |
+ |
+ |
+ |
+ |
|
VTE treatment |
+ |
+ |
+ |
+ |
|
|
Primary VTE prevention |
+ |
+ |
+ |
||
|
Recurrent VTE prevention |
+ |
+ |
+ |
||
|
CAD |
+ |
||||
|
PAD |
+ |
||||
|
Pediatric patients |
+ |
+ |
|||
|
Mechanism of action |
Direct thrombin inhibition |
Selective FXa inhibition |
|||
|
Half-life (h) |
12–17 |
Pediatric: 1.6–4.2 |
12 |
10–14 |
|
|
Adults: 5–9 |
|||||
|
Elderly: 11–13 |
|||||
|
Renal clearance/excretion (%) |
80 |
36 |
27 |
50 |
|
Table 1. Indications, mechanisms, and pharmacokinetic properties of DOACs.
Abbreviations: CAD, coronary artery disease; NVAF, nonvalvular atrial fibrillation; PAD, peripheral artery disease; VTE, venous thromboembolism.
Despite the significant improvement in anticoagulant administration, DOACs have introduced challenges for both clinical laboratorians and clinicians. Specifically, providers need to understand the impact DOACs have on diagnostic hemostasis testing. This comprehension is vital to accurately interpreting results, forming clinical impressions, and rendering sound management decisions. This document is an expert opinion and serves to provide evidence-based guidance for coagulation testing in patients who are taking DOACs. In preparing this guidance document, the focus was on the laboratory methods as the driving principle for our approach while also extracting and highlighting the most practical information in the literature to answer the following questions:
- What is the impact of DOACs on basic hemostasis testing?
- Which coagulation tests can be performed while a patient is on a DOAC?
- Which coagulation tests should be avoided while a patient is on a DOAC?
- What is the role for point-of-care (POC) testing in the presence of DOACs?
- What strategies can be used to mitigate testing interferences in patients taking DOACs?
- Should DOAC concentrations be monitored?
- What tests are available for measuring DOACs?
- How should DOAC concentrations be interpreted?
What is the impact of DOACs on basic hemostasis testing?
Basic hemostasis testing commonly consists of the prothrombin time (PT), activated partial thromboplastin time (aPTT), and fibrinogen activity assays. These assays rely on the principle that a clot can be formed due to appropriate thrombin generation. In vivo, DOACs affect the common coagulation pathway that ultimately disrupts thrombin generation (Fig. 1) and could hypothetically impact clot-based PT and aPTT testing. From a laboratory perspective, however, this is much more complicated.
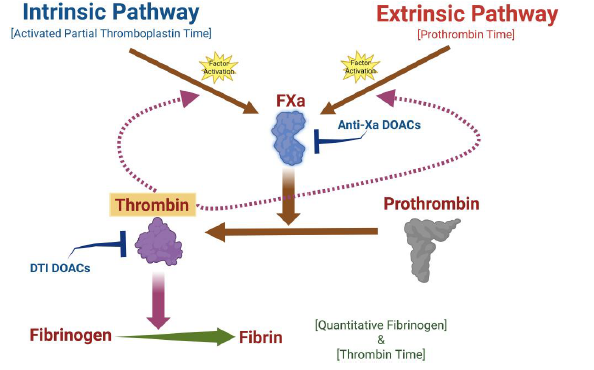
Fig. 1. A schematic illustrating the coagulation cascade in vitro. The intrinsic and extrinsic pathways intercept at FXa, which is the start of the common pathway. FXa is responsible for activating prothrombin to thrombin, and thrombin cleaves fibrinogen into fibrin, which arranges to form the fibrin clot. The anti-Xa DOACs inhibit the activity of FXa, while the thrombin inhibitor DOAC inactivates thrombin. Created in BioRender. Harris, N. (2024) https://BioRender.com/c54d428.
If the PT and/or aPTT are prolonged due to DOACs, the degree of their prolongation cannot be used to infer DOAC concentrations because of an unsuitable correlation (3–6). Further, there is variable sensitivity in testing to different DOACs that adds another layer of complexity. For example, the PT is less sensitive to apixaban than it is to rivaroxaban, and most PT reagents cannot adequately detect peak and trough levels of apixaban. On the other hand, whether DOACs will affect the PT or aPTT depends on the testing platform (i.e., the specific method, reagent lot-lot variation), the specific DOAC, drug metabolism, and drug plasma concentration (3, 5, 7, 8). Fibrinogen activity testing using the Clauss fibrinogen method is generally considered to be insensitive to DOACs, though high dabigatran concentrations may cause a false decrease for some assay reagents.
Summary
- PT and aPTT may be affected by DOACs (platform/assay dependent) and should not be used to assess DOAC presence and/or concentration.
- Fibrinogen activity measurements can generally be obtained without interference from DOACs.
WHICH COAGULATION TESTS CAN BE PERFORMED WITHOUT INTERFERENCE FROM DOACS?
The impact of DOACs varies based on the methodology used for the test. In general, methodologies not affected by the presence of DOACs include polymerase chain reaction, immunoassays, and platelet function testing. Polymerase chain reaction testing is commonly used for the detection of factor V Leiden and prothrombin gene mutations. Serology-based methods such as enzyme-linked immunosorbent assays, lateral flow immunoassays, agglutination methods, and chemiluminescent immunoassays do not rely on any type of clot formation and are unaffected by DOACs. Immunoassays are commonly used for measuring von Willebrand factor, antiphospholipid antibodies, D-dimer, protein S and C antigen, antithrombin antigen, and fibrinogen antigen.
Platelet function testing (PFT) availability varies widely between laboratories. Further, PFT can be performed on POC systems (e.g., PFA-100/200, VerifyNow™ etc.) or considered high-complexity testing (e.g., light transmission aggregometry) depending on the testing platform and method. Because PFTs do not generally rely on thrombin generation for their evaluation of platelets, they should not be affected by DOACs. The current evidence, albeit relatively limited, indicates that DOACs do not significantly affect PFT platforms (9–14). However, regarding light transmission aggregometry, specific reagent agonists used to induce platelet aggregation may be impacted by DOACs—specifically, thrombin and thrombin receptor-activating peptides (9, 10, 14–19).
Summary
- Polymerase chain reaction and immunoassays are unaffected by DOACs.
- Based on the current evidence, platelet function testing is relatively unaffected by DOACs, but specific agonists (thrombin and thrombin receptor-activating peptides) should be avoided for light transmission aggregometry.
WHICH COAGULATION TESTS SHOULD BE AVOIDED WHEN A PATIENT IS TAKING A DOAC?
In general, certain clot-based tests and some chromogenic assays should be avoided in patients taking a DOAC, as DOACs can lead to test result misinterpretation.
Should factor activity assays be avoided?
Coagulation factor activities are commonly measured using clot-based or one-stage assays. Briefly, the patient plasma is diluted with factor-deficient plasma and assayed by the PT (for factors II, V, VII, and X) or aPTT (for factors VIII, IX, XI, and XII). The measured clotting time is inversely proportional to factor activity. As described earlier, DOACs variably affect the PT and aPTT, with the degree of prolongation dependent on the assay reagent, type, and concentration of DOAC present. Therefore, factor activities determined via one-stage assays with DOAC-susceptible PT or aPTT reagents have the potential to underestimate factor activities in the presence of DOACs.
In vitro spiking studies have demonstrated that increasing concentrations of dabigatran lower PT- and especially aPTT-based factors in a concentration-dependent manner (20, 21). Of the PT-based factors, factor II may be the most affected and factor X the least affected. Misleading results can occur with dabigatran-containing samples as they often display results suggestive of a nonspecific inhibitor effect.
Increasing concentrations of rivaroxaban and apixaban can also lead to factitiously underestimated one-stage factor activities (22–25). Rivaroxaban generally has more of an effect than apixaban, which reflects the varying sensitivities of PT and aPTT reagents to these different FXa inhibitors. As with dabigatran, apixaban and rivaroxaban often induced a nonspecific inhibitor effect.
In addition to falsely lowering factor activities measured by one-stage assays, DOACs may lead to the factitious presence of factor inhibitors (20, 24).
Summary
- Factor activities measured by one-stage or clot-based methods can be underestimated in the presence of DOACs.
- Factor inhibitors measured by one-stage or clot-based methods can be overestimated in the presence of DOACs.
Should lupus anticoagulant assays be avoided?
Lupus anticoagulants (LA) are autoimmune heterogeneous immunoglobulins directed against phospholipid-protein components of the cell membrane that may impact coagulation-related proteins and/or clotting factors in vivo. If these immunoglobulins are present in a sample and bound to their targeted antigen, they may cause prolongation of phospholipid-dependent clotting times in laboratory assays. Currently, it is recommended that LA testing should employ 2 phospholipid-dependent clotting time assays based on different principles (26). Although LA testing is only 1 of the 3 laboratory criteria for the identification of antiphospholipid syndrome, it is the best-established risk factor of clinical manifestations (27–29). It is important for healthcare providers to understand the effect of DOACs in LA testing for appropriate testing utilization, diagnosis, and management.
Similar to other clot-based assays, LA tests are vulnerable to interferences by anticoagulant therapy that usually leads to false positives but in rare scenarios may cause false negatives (26, 29). Generally, DOACs have been widely shown to prolong conventional LA screening assays (3, 29, 30). This may potentially lead to misleading results and misinterpretation.
Summary
- The presence of DOACs may lead to unreliable LA test results such as false positives and, in rare cases, false negatives.
Should other clot-based testing be avoided?
Many specialized coagulation assays also rely on clot formation as a measurement endpoint and are at higher risk for interference from DOACs. A summary of the clot-based assays, their associated conditions, and susceptibilities to DOAC interference is presented in Table 2.
|
Test |
Testing indication |
FXa inhibitor interference? |
DTI interference? |
|
PT/aPTT and mixing study |
Screening test |
Yes |
Yes |
|
Thrombin time |
Screening test |
No |
Yes |
|
Fibrinogen activity |
Screening test |
No |
No |
|
Factor activities |
Hemophilia and other factor deficiencies |
Yes |
Yes |
|
Activated protein C resistance |
Thrombophilia |
Yes |
Yes |
|
Protein S activity |
Thrombophilia |
Yes |
Yes |
|
Protein C activity |
Thrombophilia |
Yes |
Yes |
|
Dilute thrombin time |
Anticoagulation |
No |
Yes |
|
Reptilase time |
Hypofibrinogenemia or dysfibrinogenemia |
No |
No |
|
Lupus anticoagulant |
Thrombophilia |
Yes |
Yes |
Clot-based protein C activity. Protein C activity via clot-based assay is overestimated in the presence of rivaroxaban, edoxaban, or dabigatran but not apixaban (31, 32). Clot-based methods are generally discouraged in favor of chromogenic assays unless there is a strong clinical suspicion for the rare type II protein C deficiency (33). False elevation in protein C activity could mask true deficiency of this natural anticoagulant, delaying appropriate diagnosis and management. It is recommended that chromogenic protein C activity testing be used in the presence of DOACs.
Protein S activity. Functional testing for protein S activity can be performed via PT-, aPTT-, or Russell viper venom time (RVVT)-based clotting tests (34). aPTT- and RVVT-based protein S activity assays are exquisitely sensitive to dabigatran (20, 35). Clot-based protein S activity may also be overestimated by FXa inhibitors and is particularly sensitive to rivaroxaban (31, 36–38). False elevation in protein S activity could mask true deficiency of this natural anticoagulant, delaying appropriate diagnosis and management. It is recommended that immunoassay-based free protein S antigen testing be used in the presence of DOACs.
Activated protein C resistance (APCR). APCR assays rely on clot formation with aPTT-, RVVT-, or prothrombinase-based reagents where the patient plasma is prediluted in factor V-deficient plasma, analyzed with and without the addition of activated protein C and a ratio between these 2 clotting times calculated. Increasing APCR ratios have been observed with increasing concentrations of dabigatran, apixaban, rivaroxaban, and edoxaban, which could lead to a false normal APCR ratio in an individual who carries the factor V Leiden mutation (20, 22, 31, 35, 39). Different APCR reagents demonstrate variable susceptibility to DOAC interference, with prothrombinase- and RVVT-based assays more affected by dabigatran and aPTT-based assays less affected by apixaban (37, 39, 40). It is recommended that molecular testing be used to test for the factor V Leiden mutation in the presence of DOACs (41).
Thrombin time (TT)/dilute TT. The TT is unaffected by FXa inhibitors but is too sensitive to dabigatran to give any indication of drug concentration (21, 31, 42). However, a modification of the thrombin time, the dilute TT, demonstrates a concentration-dependent prolongation of the clotting time and, thus, is more appropriate for dabigatran quantification (43).
Reptilase time. Reptilase time measures fibrin formation following fibrinogen cleavage and fibrinopeptide A release by the batroxobin snake venom. The reptilase test is unaffected by all DOACs (20, 22, 31, 42).
Summary
- Clot-based protein C and protein S activity may be overestimated in the presence of DOACs, potentially masking true deficiency of these natural anticoagulants.
- APCR testing is susceptible to interference from DOACs, but the interference is variable depending on the reagent and anticoagulant.
- The TT and dilute TT are prolonged by dabigatran but unaffected by FXa inhibitors.
Are chromogenic assays impacted?
Relative to clot-based assays, chromogenic assays are less sensitive to interferences from DOACs. Chromogenic assays comprise 2 stages that are both independent of clot formation: activation of a serine protease capable of cleaving a specific peptide nitroanilide substrate and cleavage of the substrate to release a chromophore whose concentration is measured spectrophotometrically. Active forms of protein C, factor II, and factor X are commonly used in chromogenic reactions.
Chromogenic protein C activity. Chromogenic protein C activity assays can be reliably performed in patients taking any DOAC, since protein C is not the target of these anticoagulants and is activated directly via viper venom (21, 24, 32).
Antithrombin activity based on factor IIa. It is important to recognize that chromogenic antithrombin activity assays that rely on factor IIa (i.e., thrombin) for chromophore generation can be performed in patients taking FXa inhibitors but will be falsely elevated in patients taking dabigatran (31). Dabigatran affects thrombin-based antithrombin assays in a dose-dependent manner (20, 21, 32, 35).
Antithrombin activity based on factor Xa. Chromogenic antithrombin activity assays commonly use factor X activity for chromophore generation and can be performed in patients taking dabigatran but will be unreliable in patients taking FXa inhibitors (20, 31, 32). Different FXa-based antithrombin reagents may demonstrate varying sensitivities to the FXa inhibitors (31).
Anti-Xa activity. The use of chromogenic anti-Xa assays for therapeutic monitoring of unfractionated heparin and low molecular weight heparin is now commonplace, but the measured activity reflects all anti-Xa activity in the specimen and will be elevated in the presence of FXa inhibitors. Therefore, anti-Xa activity assays are not reliable for heparin monitoring in patients with residual direct FXa inhibitor present (44, 45).
Chromogenic factor VIII, IX and X activities. FXa inhibitors will reduce factor VIII, IX, and X activities when measured by chromogenic principles since these assays rely on factor Xa activity for chromophore generation. Plasma apixaban and rivaroxaban concentrations well within the typical on-therapy ranges for these anticoagulants may be sufficient to yield a factitiously low chromogenic factor VIII activity (31).
Factor XIII. Dabigatran can lead to falsely low levels in the chromogenic FXIII assay (6).
|
Test |
Testing indication |
FXa inhibitor interference? |
DTI interference? |
|
Antithrombin activity (FXa-based) |
Thrombophilia |
Yes |
No |
|
Antithrombin activity (FIIa-based) |
Thrombophilia |
No |
Yes |
|
Protein C activity |
Thrombophilia |
No |
No |
|
Factor VIII |
Hemophilia A |
Yes |
No |
|
Factor X |
Anticoagulation |
Yes |
No |
|
Anti-Xa activity |
Anticoagulation |
Yes |
No |
|
Factor IX |
Hemophilia B |
Yes |
No |
|
Factor XIII |
Bleeding disorder |
No |
Yes |
Abbreviations: DTI, direct thrombin inhibitor.
Table 3. Summary of chromogenic assays.
Summary
- Chromogenic protein C assays are unaffected by DOACs.
- Chromogenic assays utilizing factor IIa for chromophore generation are affected by dabigatran but unaffected by FXa inhibitors.
- Chromogenic assays utilizing factor Xa for chromophore generation are affected by FXa inhibitors but unaffected by dabigatran.
WHAT IS THE ROLE FOR POC TESTING IN THE PRESENCE OF DOACS?
POC testing is available for several laboratory tests, such as the international normalized ratio, activated clotting times, and viscoelastic tests. POC international normalized ratios can be imprecise, vary from their central laboratory counterparts depending on the POC device, and are potentially subject to interferences (importantly, such as the presence of a lupus anticoagulant). Prolongation of international normalized ratios may be seen with a DOAC present, but a normal result also does not exclude the presence of a DOAC either. Currently, there is no role for POC testing for monitoring the therapeutic effects of DOACs.
Viscoelastic testing
The use of viscoelastic testing to measure DOAC effects is not recommended. The rationale for this is a lack of specificity of alterations to the coagulation cascade. Another consideration is the relatively wide reference range of the viscoelastic methods. The major effect of the DOACs is a prolongation of the initial clotting reaction time, which reflects the coagulation cascade and the generation of thrombin (46–51). This interval is the R time (thromboelastography analyzer) or the CT (ROTEM and Quantra analyzers). In many reports, these reaction times, especially for the direct FXa inhibitors, are still within the population-based reference range even though they are prolonged above an individual patient’s baseline reaction time. Apixaban has the least discernable effect on the clot or reaction time, while edoxaban has the greatest effect. It appears that dabigatran is more likely to prolong these initial reaction times beyond the reference range. Significant prolongation of the R or CT by direct FXa inhibitors often requires supratherapeutic doses of this class of DOACs (52). Overall, the results of these prior studies demonstrate various and inconsistent effects of DOACs on viscoelastic testing.
Activated clotting time
The activated clotting time is a POC whole blood assay used to monitor heparin anticoagulation in cardiopulmonary bypass, percutaneous coronary intervention, and extracorporeal membrane oxygenation (53, 54). The blood specimen is mixed with kaolin or silica, which activates the intrinsic pathway of coagulation. Clotting is detected by the movement of a magnet or photo-optically in a cartridge by the change in velocity of blood as the viscosity increases. Only dabigatran significantly prolongs the activated clotting time. The direct FXa inhibitors do not have a significant effect on the activated clotting; therefore, heparin monitoring by activated clotting time can be performed in the presence of FXa inhibitors (55).
Summary
- POC testing does not currently have a role in monitoring the therapeutic effects of DOACs.
- DOACs can demonstrate inconsistent effects on viscoelastic testing.
- Direct thrombin inhibitors can prolong activating clotting times, but the direct FXa inhibitors do not have a significant effect.
WHAT STRATEGIES CAN BE USED TO MITIGATE TESTING INTERFERENCES IN PATIENTS TAKING DOACS?
There are 3 general strategies that can be implemented to mitigate these known interferences, which include (a) discontinuing DOAC treatment, (b) using a different analytical method not subject to the interference, and (c) reversal or removal agents to neutralize the inhibition. In situations where testing must be performed while patients are prescribed DOACs, temporary discontinuation of the DOAC ideally should be considered using appropriate clinical judgment. Additionally, not all DOACs behave in the same manner in specific assays, and a change in methodology may be a helpful alternative. For example, the chromogenic antithrombin activity assay is available in 2 different forms; one form uses the inhibition of FXa, and the other uses the inhibition of thrombin. Direct FXa inhibitors interfere with assays using the inhibition of exogenous FXa, causing falsely increased results for antithrombin (56). In contrast, antithrombin assays that use exogenous thrombin inhibition do not show any interference from direct FXa inhibitors. The following discussion focuses on other strategies that may mitigate DOAC interference in specific assays, including a section on specific products for DOAC neutralization.
Strategies for factor assays
The underestimation of factor activity and nonspecific inhibitor effect seen in clot-based factor assays (see section 3a) may be mitigated by multidilution analysis and utilizing DOAC-insensitive reagents (32). However, these strategies have been shown to be potentially more effective for rivaroxaban and apixaban than for dabigatran.
Strategies for LA testing
Similarly to other coagulation testing, it is preferable for LA testing to be performed in the absence of anticoagulants (i.e., before the initiation of DOAC therapy) (26, 29). If therapy has been started and testing is required, an earnest consideration of temporarily stopping therapy should be evaluated. If the risk of temporary discontinuation is not excessively high, consider stopping the DOAC therapy for a minimum of 2 to 3 days before collecting the sample for LA testing (3, 28). If the risk of temporary discontinuation of anticoagulation is too high, consider a temporary transition to low molecular weight heparin, which may have less interference on the LA testing, and draw trough levels just before the next dose (3).
Strategies for DOAC neutralization
If anticoagulant discontinuation is not safe or feasible, consider the use of DOAC removal agents in sample preparation prior to performing coagulation testing. DOAC-STOPTM and DOAC-RemoveTM are products that claim to absorb or remove any DOAC in plasma samples while having a minimal effect on plasma proteins involved in the clotting mechanism and are supported by some studies such as PT, aPTT, anti-Xa, TT, and LA panels (57–61). More recently, a DOAC filter has been introduced to remove DOACs prior to LA testing (62). These assays are classified as laboratory-developed tests, which require additional resources and a thorough evaluation by the laboratory director and staff.
Similarly, the reversal agents idarucizumab and andexanet alfa may be used as a sample pretreatment step to neutralize the impact of dabigatran and factor Xa inhibitors, respectively. Idarucizumab is a monoclonal antibody fragment that binds to dabigatran with an affinity around 350 times stronger than its affinity for thrombin (63). Andexanet alfa is a recombinant modified human factor Xa decoy protein that has no catalytic activity but can bind factor Xa inhibitors with high affinity (64). Both have been shown to neutralize DOACs in samples prior to coagulation testing (65, 66).
Importantly, the approach with DOAC neutralizing agents can be cost-prohibitive and not feasible for all laboratories. Utilizing these strategies requires resources and direct discussions with laboratory leadership for an evaluation and implementation
Communication strategies for laboratories
In most cases, the strategies discussed here require communication between the laboratory and clinicians. The laboratorians have information regarding the potential interferences by DOACs on the laboratory assays, while clinicians are aware of their patient’s most current clinical context, including anticoagulation status, future anticoagulation management, and laboratory testing needs. Effective communication, exchange of information, and crafting strategies between both parties have been shown to optimize patient care (67). The level of communication between parties will likely vary and be dependent on the type of medical institution and laboratory. A simple route achievable for many laboratories could include attaching a comment to results where certain DOACs could interfere. An example is attaching a comment that reads, “Factor Xa inhibitors may lead to false increases in this test” to an antithrombin assay that uses factor Xa. A higher level of communication could include continuing medical education lectures given by the laboratory director or staff to ordering providers and/or on an individual case-by-case basis if the laboratory director is involved in coagulation test interpretations and direct communication with the clinical team. Another method could incorporate information technology to guide ordering based on a defined algorithmic approach to ultimately tailor the best available testing for the patient’s specific scenario. The aforementioned strategies may be used in combinations or in hybrid forms to satisfy individual institutional needs.
Summary
- Alternative laboratory testing methods may be considered to assist with interpreting coagulation test results when DOAC interference is involved.
- Clot-based factor and inhibitor testing should be avoided in patients taking DOACs, but if testing must be performed, multidilution analysis and use of DOAC-insensitive PT and aPTT reagents are recommended if available.
- LA testing should be performed in the absence of DOAC treatment, but if testing is necessary, multiple strategies should be considered by both the clinical and the laboratory team in collaboration.
- DOAC neutralizing reagents are available and may have some potential for their utilization. However, they are cost-prohibitive and may not be possible to implement by most laboratories.
- Multiple strategies exist for laboratories to communicate with clinicians about the impact of DOACs on coagulation testing.
SHOULD DOAC CONCENTRATIONS BE MONITORED?
DOACs have predictable pharmacokinetics and pharmacodynamics relative to warfarin with fewer interferences from food and other medications; therefore, routine monitoring is generally not necessary for dose adjustments, especially when there are no clinical concerns for toxicity or lack of efficacy (5, 7, 68–70).
There are urgent and nonurgent situations proposed where screening for the presence of DOACs may be clinically useful. Urgent situations include severe hemorrhage (toxicity) or thrombosis (therapy failure), an emergent surgical procedure while on therapy, and trauma. Nonurgent situations include advanced age, severe renal failure with dialysis dependence, prior intervention with high bleeding risk, prior small bowel resection, gastrointestinal malabsorption, patients with extremes of body weight, failed therapy, and drug interactions (9, 71–74).
It is highly discouraged to test patients beyond these very limited clinical scenarios based on the available data (75). Accordingly, due to the limited clinical utility, infrequent need, and relatively predictable pharmacokinetics and pharmacodynamics, many clinical laboratories do not perform assays that measure DOACs.
Summary
- Patients on DOACs do not require routine laboratory monitoring, though there may be very limited and specific urgent or nonurgent situations where DOAC plasma concentration measurements may help guide clinical decision-making.
WHAT TESTS ARE AVAILABLE FOR MEASURING DOACS?
If DOAC measurement is being considered, there are indirect and direct measurements that can be strategically implemented. While widely available, the PT and aPTT are not appropriate for measuring DOACs.
Indirect measurement
Indirect DOAC assays are used to determine the presence or absence of the drug in the plasma of the patient (5, 7, 76, 77). TT is highly sensitive to dabigatran and can be used to detect the presence of dabigatran. A normal TT rules out the presence of dabigatran in a specimen. Note the TT is not affected by the FXa inhibitors. In contrast, normal PT and/or aPTT results cannot be used to definitively exclude the presence of a DOAC in a sample (3–6).
Chromogenic anti-Xa assay calibrated for unfractionated heparin and low molecular weight heparin can detect the presence of direct FXa inhibitors (7). Since anti-Xa assays are widely available, they may be useful in emergency situations to detect the presence of a factor Xa inhibitor in the patient’s plasma, though they demonstrate variable sensitivity to FXa DOACs and will not provide a DOAC concentration (78). This assay is widely available and may assist with DOAC detection (7).
Direct DOAC assays measure the drug concentration usually in ng/mL (5, 7, 76, 77). For direct measurements, LC-MS/MS is considered the gold standard. Each assay is calibrated with each drug to be measured, and this method demonstrates good accuracy and precision over a broad concentration range, although it is not widely available (79). At this time, there are no international reference materials available. Peak and trough measurements are preferred as they give more information than random measurements (80). This method cannot be used in emergency settings.
Chromogenic anti-Xa assays that use the appropriate direct FXa inhibitor calibrators and controls can be used to measure drug levels with a relatively short turnaround time compared to LC-MS/MS. They can be performed in as little as 30 min in some laboratories and may eliminate the need to use reversal agents or other hemostatic agents in patients with little or no anti-Xa activity (81).
Ecarin-based assays (clot based and chromogenic) provide a direct measure of dabigatran activity. This assay is not readily available or useful in the absence of specific kits and standards (i.e., standardization of the concentration of ecarin in the test). It has been demonstrated to have a good correlation with LC-MS/MS-based methods (5, 7, 76).
The chromogenic anti-FIIa method is a quantitative measurement of dabigatran and other direct thrombin inhibitors and is based on the inhibition of a constant and defined quantity of thrombin. The method has good correlation with LC-MS/MS-based methods (7, 82, 83). The dilute TT using equal volumes of diluted patient plasma and thrombin with dabigatran calibrators is suitable for quantitative assessment of the anticoagulant effect of dabigatran (7, 76). A strong correlation between dilute TT and LC-MS/MS has been reported (84).
Summary
- There are indirect and direct methods that are available for measuring DOACs.
- The TT can be used to rule out dabigatran, and dilute TT and ecarin-based assays can measure dabigatran concentrations.
- Heparin-calibrated anti-Xa assays can detect the presence of direct FXa inhibitors, but calibration with specific drugs is required to provide direct FXa inhibitor concentrations.
- LC-MS/MS is the gold standard for measurement of DOACs.
HOW SHOULD DOAC CONCENTRATIONS BE INTERPRETED?
Many factors may affect DOAC concentrations, including drug–drug interactions, renal dysfunction, prior bariatric surgery, concomitant food intake in certain scenarios (e.g., therapeutic doses of rivaroxaban), and dose intensity. At the current time, standardized therapeutic ranges for DOACs have not been established. Peak and trough “on-therapy” plasma concentrations were observed in various pharmacokinetic and pharmacodynamic studies, but therapeutic reference ranges have not been established (85, 86).
Given the lack of robust data that DOAC concentrates significantly change management decisions, guidelines by the International Society of Thrombosis and Haemostasis Scientific and Standardization Committee suggest not routinely monitoring DOACs concentration (87). However, there may be clinical scenarios where measuring DOAC concentrations could potentially be useful. For example, these guidelines suggest measuring a trough level when using DOACs after bariatric surgery (no sooner than 4 weeks postoperatively) to provide insight regarding drug absorption. Another scenario where measuring DOAC concentrations might be helpful is in the setting of a major or life-threatening bleed. Prothrombin complex concentrates, andexanet alfa, and idarucizumab are available reversal agents for FXa inhibitors and dabigatran. International Society of Thrombosis and Haemostasis Scientific and Standardization Committee guidelines from 2016 and 2024 suggested that FXa inhibitor concentrations greater than 50 ng/mL in the setting of severe bleeding are likely sufficient to warrant anticoagulant reversal. The threshold can be lowered to 30 ng/mL in life-threatening bleeding situations (73, 88).
Yet, even in these limited scenarios, the challenge is that there are no clear optimal “cutoff” plasma concentrations for a trough level after bariatric surgery or for using emergent reversal agents with serious bleeding. Additionally, few clinical laboratories offer rapid assays for DOAC concentrations, further limiting the realistic utilization of a cutoff concentration for emergent reversal. Ultimately, appropriate DOAC reversal in the setting of major bleeding depends on multiple factors, including timing of the most recent DOAC dose and dose intensity.
Summary
- There are currently no universal or standardized therapeutic ranges of DOAC plasma concentrations. DOAC plasma concentrations may be used in certain scenarios, including emergent anticoagulant reversal, but the optimal cutoff concentration has yet to be determined.
Conclusion
The introduction of DOACs has brought major improvements in anticoagulation management. The oral route of administration with fixed doses along with no requirement for routine therapeutic monitoring has dramatically improved clinical care since the early 2010s. The now ubiquitous use of DOACs, however, poses challenges in understanding impacts on coagulation testing. This document explores these impacts to provide guidance to both the laboratory and clinical sides of patient care.
Clinicians ordering coagulation testing for patients taking DOACs should approach these scenarios thoughtfully, as this requires discretion to determine appropriate and optimal timing for testing. Communication and collaboration with the laboratory leadership and staff is strongly suggested prior to testing. The laboratory medical director can provide guidance to the clinical team on current methodologies and how to interpret results for patients on DOACs.
Nonstandard Abbreviations: DOAC, direct oral anticoagulant; FXa, factor Xa; POC, point of care; PT, prothrombin time; aPTT, activated partial thromboplastin time; PFT, platelet function testing; LA, lupus anticoagulants; RVVT, Russell viper venom time; APCR, activated protein C resistance; TT, thrombin time.
Author Contributions: The corresponding author takes full responsibility that all authors on this publication have met the following required criteria of eligibility for authorship: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved. Nobody who qualifies for authorship has been omitted from the list.
Lindsay Bazydlo (Conceptualization-Equal, Writing—original draft-Equal, Writing—review & editing-Equal), Maximo Marin (Conceptualization-Equal, Writing—original draft-Equal, Writing—review & editing-Equal), Anna Merrill (Conceptualization-Equal, Writing—original draft-Equal, Writing—review & editing-Equal), Louise M. Man (Conceptualization-Equal, Writing—original draft-Equal, Writing—review & editing-Equal), Olajumoke Oladipo (Conceptualization-Equal, Writing—original draft-Equal, Writing—review & editing-Equal), and Neil Harris (Conceptualization-Equal, Writing—original draft-Equal, Writing—review & editing-Equal)
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form.
Research Funding: None declared
Disclosures: L.A.L. Bazydlo received an honorarium, travel support, and conference registration for presentation of this work at ADLM 2025. O.O. Oladipo received honoraria from Diagnostica Stago, Inc. A.E. Merrill has received consulting fees from Diagnostica Stago, Inc., an honorarium from ADLM, an honorarium from the Association for Molecular Pathology; is on an advisory board for Diagnostica Stago, Inc.; is on the editorial board for Annals of Laboratory Medicine; and is an associate editor for The Journal of Applied Laboratory Medicine, ADLM.
Role of Sponsor: No sponsor was declared.
REFERENCES
- Becattini C, Vedovati MC, Agnelli G. Old and new oral anticoagulants for venous thromboembolism and atrial fibrillation: a review of the literature. Thromb Res 2012;129:392–400.
- Wittkowsky AK. Novel oral anticoagulants and their role in clinical practice. Pharmacotherapy 2011;31:1175–91.
- Moser KA, Smock KJ. Direct oral anticoagulant (DOAC) interference in hemostasis assays. Hematology Am Soc Hematol Educ Program 2021;2021:129–33.
- Adcock DM, Gosselin RC. The danger of relying on the APTT and PT in patients on DOAC therapy, a potential patient safety issue. Int J Lab Hematol 2017;39(Suppl 1):37–40.
- Dunois C. Laboratory monitoring of direct oral anticoagulants (DOACs). Biomedicines 2021;9:445.
- Gosselin RC, Adcock DM, Douxfils J. An update on laboratory assessment for direct oral anticoagulants (DOACs). Int J Lab Hematol 2019;41(Suppl 1):33–9.
- Gosselin RC, Adcock DM, Bates SM, Douxfils J, Favaloro EJ, Gouin-Thibault I, et al. International council for standardization in haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost 2018;118:437–50.
- Kim PY, Yeh CH, Dale BJ, Leslie BA, Stafford AR, Fredenburgh JC, et al. Mechanistic basis for the differential effects of rivaroxaban and apixaban on global tests of coagulation. TH Open 2018;2:e190–201.
- Douxfils J, Adcock DM, Bates SM, Favaloro EJ, Gouin-Thibault I, Guillermo C, et al. 2021 update of the international council for standardization in haematology recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost 2021;121:1008–20.
- Eller T, Busse J, Dittrich M, Flieder T, Alban S, Knabbe C, Birschmann I. Dabigatran, rivaroxaban, apixaban, argatroban and fondaparinux and their effects on coagulation POC and platelet function tests. Clin Chem Lab Med 2014;52:835–44.
- Gosselin B, Adcock D. Direct oral anticoagulants: impact and interference of DOACs on coagulation testing. https://www.myadlm.org/science-and-research/clinical-chemistry-trainee-council/trainee-council-in-english/pearls-of-laboratory-medicine/2020/direct-oral-anticoagulants-impact-and-interference-of-doacs-on-coagulation-testing (Accessed May 2024).
- Hall RP, Majumdar M, Ferreira SS, Lee I, Bellomo T, Jessula S, et al. Impact of factor Xa inhibition on coagulation, platelet reactivity, and thrombosis in patients with peripheral artery disease. Ann Vasc Surg 2023;97:211–20.
- Jourdi G, Bachelot-Loza C, Mazoyer E, Poirault-Chassac S, Duchemin J, Fontenay M, Gaussem P. Effect of rivaroxaban and dabigatran on platelet functions: in vitro study. Thromb Res 2019;183:159–62.
- Vinholt PJ, Nielsen C, Soderstrom AC, Brandes A, Nybo M. Dabigatran reduces thrombin-induced platelet aggregation and activation in a dose-dependent manner. J Thromb Thrombolysis 2017;44:216–22.
- Nehaj F, Sokol J, Ivankova J, Mokan M, Kovar F, Stasko J, Mokan M. First evidence: TRAP-induced platelet aggregation is reduced in patients receiving xabans. Clin Appl Thromb Hemost 2018;24:914–9.
- Nehaj F, Sokol J, Ivankova J, Mokan M, Mokan M, Stasko J. Edoxaban affects TRAP-dependent platelet aggregation. J Thromb Thrombolysis 2020;49:578–83.
- Nehaj F, Sokol J, Mokan M, Ivankova J, Mokan M. Thrombin receptor agonist peptide-induced platelet aggregation is reduced in patients receiving dabigatran. Clin Appl Thromb Hemost 2018;24:268–72.
- Olivier CB, Weik P, Meyer M, Weber S, Anto-Michel N, Diehl P, et al. TRAP-induced platelet aggregation is enhanced in cardiovascular patients receiving dabigatran. Thromb Res 2016;138:63–8.
- Sokol J, Nehaj F, Ivankova J, Mokan M, Lisa L, Zolkova J, et al. Impact of edoxaban on thrombin-dependent platelet aggregation. Clin Appl Thromb Hemost 2020;26:1076029620948585.
- Adcock DM, Gosselin R, Kitchen S, Dwyre DM. The effect of dabigatran on select specialty coagulation assays. Am J Clin Pathol 2013;139:102–9.
- Bonar R, Favaloro EJ, Mohammed S, Pasalic L, Sioufi J, Marsden K. The effect of dabigatran on haemostasis tests: a comprehensive assessment using in vitro and ex vivo samples. Pathology 2015;47:355–64.
- Douxfils J, Chatelain C, Chatelain B, Dogne J-M, Mullier F. Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost 2013;110:283–94.
- Scheres LJJ, Lijfering WM, Middeldorp S, Cheung YW, Barco S, Cannegieter SC, Coppens M. Measurement of coagulation factors during rivaroxaban and apixaban treatment: results from two crossover trials. Res Pract Thromb Haemost 2018;2:689–95.
- Bonar R, Favaloro EJ, Mohammed S, Ahuja M, Pasalic L, Sioufi J, Marsden K. The effect of the direct factor Xa inhibitors apixaban and rivaroxaban on haemostasis tests: a comprehensive assessment using in vitro and ex vivo samples. Pathology 2016;48:60–71.
- Gerotziafas GT, Baccouche H, Sassi M, Galea V, Chaari M, Hatmi M, et al. Optimisation of the assays for the measurement of clotting factor activity in the presence of rivaroxaban. Thromb Res 2012;129:101–3.
- Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009;7:1737–40.
- Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306.
- Devreese KMJ, de Groot PG, de Laat B, Erkan D, Favaloro EJ, Mackie I, et al. Guidance from the scientific and standardization committee for lupus anticoagulant/antiphospholipid antibodies of the international society on thrombosis and haemostasis: update of the guidelines for lupus anticoagulant detection and interpretation. J Thromb Haemost 2020;18:2828–39.
- Tripodi A, Cohen H, Devreese KMJ. Lupus anticoagulant detection in anticoagulated patients. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2020;18:1569–75.
- Adcock DM, Gosselin R. Direct oral anticoagulants (DOACs) in the laboratory: 2015 review. Thromb Res 2015;136:7–12.
- Gosselin R, Grant RP, Adcock DM. Comparison of the effect of the anti-Xa direct oral anticoagulants apixaban, edoxaban, and rivaroxaban on coagulation assays. Int J Lab Hematol 2016;38:505–13.
- Siriez R, Dogne J-M, Gosselin R, Laloy J, Mullier F, Douxfils J. Comprehensive review of the impact of direct oral anticoagulants on thrombophilia diagnostic tests: practical recommendations for the laboratory. Int J Lab Hematol 2021;43:7–20.
- Cooper PC, Pavlova A, Moore GW, Hickey KP, Marlar RA. Recommendations for clinical laboratory testing for protein C deficiency, for the subcommittee on plasma coagulation inhibitors of the ISTH. J Thromb Haemost 2020;18:271–7.
- Marlar RA, Gausman JN, Tsuda H, Rollins-Raval MA, Brinkman HJM. Recommendations for clinical laboratory testing for protein s deficiency: communication from the SSC committee plasma coagulation inhibitors of the ISTH. J Thromb Haemost 2021;19:68–74.
- Kim YA, Gosselin R, Van Cott EM. The effects of dabigatran on lupus anticoagulant, diluted plasma thrombin time, and other specialized coagulation assays. Int J Lab Hematol 2015;37:e81–4.
- Maryamchik E, Rosenbaum MW, Van Cott EM. Rivaroxaban causes missed diagnosis of protein s deficiency but not of activated protein C resistance (factor V Leiden). Arch Pathol Lab Med 2018;142:70–4.
- Maryamchik E, Van Cott EM. Apixaban does not interfere with protein S or activated protein C resistance (factor V Leiden) testing using aPTT-based methods. Arch Pathol Lab Med 2020;144:1401–7.
- Smock KJ, Plumhoff EA, Meijer P, Hsu P, Zantek ND, Heikal NM, Van Cott EM. Protein s testing in patients with protein S deficiency, factor V Leiden, and rivaroxaban by North American specialized coagulation laboratories. Thromb Haemost 2016;116:50–7.
- Kopytek M, Zabczyk M, Malinowski KP, Undas A, Natorska J. DOAC-remove abolishes the effect of direct oral anticoagulants on activated protein C resistance testing in real-life venous thromboembolism patients. Clin Chem Lab Med 2020;58:430–7.
- Gessoni G, Valverde S, Gessoni F, Valle R. The effect of dabigatran and rivarovaban on a prothrombinase-based assay for activated protein C resistance: a preliminary study in subjects heterozygous for factor V Leiden. Blood Transfus 2015;13:666–8.
- Moore GW, Van Cott EM, Cutler JA, Mitchell MJ, Adcock DM; Subcommittee on Plasma Coagulation Inhibitors. Recommendations for clinical laboratory testing of activated protein C resistance; communication from the SSC of the ISTH. J Thromb Haemost 2019;17:1555–61.
- Douxfils J, Mullier F, Loosen C, Chatelain C, Chatelain B, Dogne J-M. Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res 2012;130:956–66.
- Avecilla ST, Ferrell C, Chandler WL, Reyes M. Plasma-diluted thrombin time to measure dabigatran concentrations during dabigatran etexilate therapy. Am J Clin Pathol 2012;137:572–4.
- May JE, Siniard RC, Taylor LJ, Marques MB, Gangaraju R. From activated partial thromboplastin time to antifactor Xa and back again. Am J Clin Pathol 2022;157:321–7.
- Strickland SW, Palkimas S, Acker M, Bazydlo LAL. A novel laboratory assay to monitor unfractionated heparin dosing in patients taking apixaban prior to hospital admission. J Appl Lab Med 2021;6:378–86.
- Henskens YMC, Gulpen AJW, van Oerle R, Wetzels R, Verhezen P, Spronk H, et al. Detecting clinically relevant rivaroxaban or dabigatran levels by routine coagulation tests or thromboelastography in a cohort of patients with atrial fibrillation. Thromb J 2018;16:3.
- Seyve L, Richarme C, Polack B, Marlu R. Impact of four direct oral anticoagulants on rotational thromboelastometry (ROTEM). Int J Lab Hematol 2018;40:84–93.
- Bai C-W, Ruan R-X, Pan S, Huang C-R, Zhang X-C, Pang Y, et al. Application of thromboelastography in comparing coagulation difference of rivaroxaban and enoxaparin for thromboprophylaxis after total hip arthroplasty. J Orthop Surg (Hong Kong) 2021;29:23094990211042674.
- Myers SP, Dyer MR, Hassoune A, Brown JB, Sperry JL, Meyer MP, et al. Correlation of thromboelastography with apparent rivaroxaban concentration: has point-of-care testing improved? Anesthesiology 2020;132:280–90.
- Kopytek M, Zabczyk M, Natorska J, Malinowski KP, Undas A. Effects of direct oral anticoagulants on thromboelastographic parameters and fibrin clot properties in patients with venous thromboembolism. J Physiol Pharmacol 2020;71:47-53.
- Iapichino GE, Bianchi P, Ranucci M, Baryshnikova E. Point-of-care coagulation tests monitoring of direct oral anticoagulants and their reversal therapy: state of the art. Semin Thromb Hemost 2017;43:423–32.
- Mehrotra S, Hoppensteadt D, Jeske W, Siddiqui F, Iqbal O, Tafur A, et al. Differential neutralization of apixaban, betrixaban, edoxaban, and rivaroxaban by andexanet alfa as measured by whole blood thromboelastographic analysis. Clin Appl Thromb Hemost 2022;28:10760296221138297.
- Bowers J, Ferguson JJ 3rd. The use of activated clotting times to monitor heparin therapy during and after interventional procedures. Clin Cardiol 1994;17:357–61.
- Kaur P, Dean C, Chu U. Coagulation, limited. Cgl-c 2021. Activated clotting time (ACT). Northfield (IL): College of American Pathologists (CAP); 2021.
- Negro F, Caravelli P, Morganti R, Casini M, Ruocco L, Tripodi A, De Caterina R. The non-vitamin K antagonist oral anticoagulants and heparin-induced prolongation of the activated coagulation time. Vascul Pharmacol 2022;144:106994.
- Hillarp A, Gustafsson KM, Faxalv L, Strandberg K, Baghaei F, Fagerberg Blixter I, et al. Effects of the oral, direct factor Xa inhibitor apixaban on routine coagulation assays and anti-FXa assays. J Thromb Haemost 2014;12:1545–53.
- Al-Qawzai Z, Dale C, Dave M, Yartey N, Platton S. Effect of DOAC-remove on coagulation screening assays in samples from patients receiving oral or parenteral anticoagulation. Int J Lab Hematol 2022;44:e95–9.
- Platton S, Hunt C. Influence of DOAC stop on coagulation assays in samples from patients on rivaroxaban or apixaban. Int J Lab Hematol 2019;41:227–33.
- Haematex. DOAC-Stop. https://www.haematex.com/haematex-products/doac-stop (Accessed May 2023).
- Skaugen JM, Sayre C, Hassett AC, Chibisov I, Bontempo F, Meyer MP, Seheult JN. Performance characteristics of DOAC-remove for neutralization of the effects of apixaban and rivaroxaban in lupus anticoagulant assays. Am J Clin Pathol 2022;157:457–69.
- Slavik L, Jacova J, Friedecky D, Ulehlova J, Tauber Z, Prochazkova J, et al. Evaluation of the DOAC-Stop procedure by LC-MS/MS assays for determining the residual activity of dabigatran, rivaroxaban, and apixaban. Clin Appl Thromb Hemost 2019;25:1076029619872556.
- Linskens EA, De Kesel P, Devreese KMJ. Direct oral anticoagulant removal by a DOAC filter: impact on lupus anticoagulant testing—evaluation on spiked and patient samples. Res Pract Thromb Haemost 2022;6:e12633.
- Schiele F, van Ryn J, Canada K, Newsome C, Sepulveda E, Park J, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 2013;121:3554–62.
- Siegal DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med 2015;373:2413–24.
- Jacquemin M, Toelen J, Feyen L, Schoeters J, Van Horenbeeck I, Vanlinthout I, et al. The adsorption of dabigatran is as efficient as addition of idarucizumab to neutralize the drug in routine coagulation assays. Int J Lab Hematol 2018;40:442–7.
- Frackiewicz A, Kalaska B, Miklosz J, Mogielnicki A. The methods for removal of direct oral anticoagulants and heparins to improve the monitoring of hemostasis: a narrative literature review. Thromb J 2023;21:58.
- Lubin IM, Astles JR, Shahangian S, Madison B, Parry R, Schmidt RL, Rubinstein ML. Bringing the clinical laboratory into the strategy to advance diagnostic excellence. Diagnosis (Berl) 2021;8:281–94.
- McRae HL, Militello L, Refaai MA. Updates in anticoagulation therapy monitoring. Biomedicines 2021;9:262.
- Hindley B, Lip GYH, McCloskey AP, Penson PE. Pharmacokinetics and pharmacodynamics of direct oral anticoagulants. Expert Opin Drug Metab Toxicol 2023;19:911–23.
- Eikelboom JW, Quinlan DJ, Hirsh J, Connolly SJ, Weitz JI. Laboratory monitoring of non-vitamin K antagonist oral anticoagulant use in patients with atrial fibrillation: a review. JAMA Cardiol 2017;2:566–74.
- Lippi G, Favaloro EJ. Recent guidelines and recommendations for laboratory assessment of the direct oral anticoagulants (DOACs): is there consensus? Clin Chem Lab Med 2015;53:185–97.
- Samama MM, Guinet C. Laboratory assessment of new anticoagulants. Clin Chem Lab Med 2011;49:761–72.
- Levy JH, Ageno W, Chan NC, Crowther M, Verhamme P, Weitz JI; Subcommittee on Control of Anticoagulation. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost 2016;14:623–7.
- Weitz JI, Eikelboom JW. Urgent need to measure effects of direct oral anticoagulants. Circulation 2016;134:186–8.
- Renon F, Rago A, Liccardo B, D'Andrea A, Riegler L, Golino P, et al. Direct oral anticoagulants plasma levels measurement: clinical usefulness from trials and real-world data. Semin Thromb Hemost 2021;47:150–60.
- Conway SE, Hwang AY, Ponte CD, Gums JG. Laboratory and clinical monitoring of direct acting oral anticoagulants: what clinicians need to know. Pharmacotherapy 2017;37:236–48.
- Douxfils J, Ageno W, Samama C-M, Lessire S, Ten Cate H, Verhamme P, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost 2018;16:209–19.
- Rimsans J, Douxfils J, Smythe MA, Gosselin RC. Overview and practical application of coagulation assays in managing anticoagulation with direct oral anticoagulants (DOACs). Curr Pharmacol Rep 2020;6:241–59.
- Schmitz EM, Boonen K, van den Heuvel DJ, van Dongen JL, Schellings MW, Emmen JM, et al. Determination of dabigatran, rivaroxaban and apixaban by ultra-performance liquid chromatography—tandem mass spectrometry (UPLC-MS/MS) and coagulation assays for therapy monitoring of novel direct oral anticoagulants. J Thromb Haemost 2014;12:1636–46.
- Douxfils J, Pochet L, Lessire S, Vancraeynest C, Dogné J-M, Mullier F. Mass spectrometry in the therapeutic drug monitoring of direct oral anticoagulants. Useful or useless? TrAC Trends in Analytical Chemistry 2016;84(Part B):41–50.
- Sarode R. Direct oral anticoagulant monitoring: what laboratory tests are available to guide us? Hematology Am Soc Hematol Educ Program 2019;2019:194–7.
- Amiral J, Dunois C, Amiral C, Seghatchian J. An update on laboratory measurements of dabigatran: smart specific and calibrated dedicated assays for measuring anti-IIa activity in plasma. Transfus Apher Sci 2016;54:428–37.
- Schmohl M, Gansser D, Moschetti V, Stangier J. Measurement of dabigatran plasma concentrations by calibrated thrombin clotting time in comparison to LC-MS/MS in human volunteers on dialysis. Thromb Res 2015;135:532–6.
- Douxfils J, Dogne JM, Mullier F, Chatelain B, Ronquist-Nii Y, Malmstrom RE, Hjemdahl P. Comparison of calibrated dilute thrombin time and aPTT tests with LC-MS/MS for the therapeutic monitoring of patients treated with dabigatran etexilate. Thromb Haemost 2013;110:543–9.
- Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet 2014;53:1–16.
- Frost C, Nepal S, Wang J, Schuster A, Byon W, Boyd RA, et al. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor Xa inhibitor, in healthy subjects. Br J Clin Pharmacol 2013;76:776–86.
- Martin KA, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost 2021;19:1874–82.
- Levy JH, Shaw JR, Castellucci LA, Connors JM, Douketis J, Lindhoff-Last E, et al. Reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost 2024;22:2889–99.

