Primary aldosteronism (PA) is a group of disorders characterized by inappropriate aldosterone production. Once considered a medical oddity, PA is now recognized as the most common cause of secondary hypertension with estimated prevalence between 5%-15% (1). The two main causes for PA are bilateral adrenal hyperplasia (BAH) and unilateral aldosterone producing adenoma (APA), which make up approximately 65%-70% and 30%-35% of all PA cases, respectively (1). Other conditions under the PA rubric include adrenal carcinoma, inherited conditions of familial hyperaldosteronism, and idiopathic hyperaldosteronism (IHA).
The first case of likely PA in the medical literature dates to 1953 and a hospitalist named MichaÅ‚ Lityn’ski (2). This initial account, published in Polish, describes a patient with symptoms of arterial hypertension and hypokalemia caused by an adrenocortical adenoma. However, this early report would go unnoticed until Jerome Conn’s groundbreaking publication of a similar case characterizing PA as the classical condition of arterial hypertension, suppressed plasma renin, increased plasma aldosterone concentrations, and resultant hypokalemia (e.g. Conn’s syndrome).
PA is a Major Public Health Concern
The 2016 Endocrine Society guidelines for PA labeled the disorder a major public health concern (1). Patients with PA have higher cardiovascular morbidity and mortality than patients with essential hypertension with similarly elevated blood pressure (BP), making it important to diagnosis these patients early.
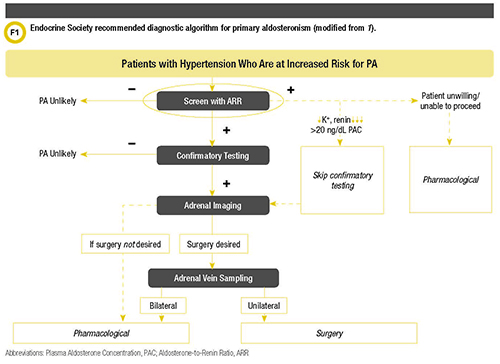
However, for physicians who do not encounter this diagnosis frequently, the diagnostic work-up can be difficult, as many patients and analytical variables need to be considered. Fortunately, the Endocrine Society guidelines offer guidance for screening high-risk patients for PA as well as a diagnostic algorithm (Figure 1).
Patients meeting one of these criteria should be screened for PA:
Sustained BP >150/100 mm Hg on each of three measurements obtained on different days.
Hypertension (BP>140/90 mm Hg) resistant to three conventional antihypertensive drugs (including a diuretic).
Controlled BP (<140/90 mm Hg) on four or more antihypertensive drugs.
Hypertension and spontaneous or diuretic-induced hypokalemia.
Hypertension and adrenal incidentaloma.
Hypertension and sleep apnea.
Hypertension and a family history of early onset hypertension or cerebrovascular accident at a young age (<40 years).
All hypertensive first-degree relatives of patients with PA.
Screening Test of Choice for Suspected PA
Any patient with one or more of these criteria should be screened initially with the aldosterone-to-renin ratio (ARR), regarded as the gold standard and most reliable screening test for PA (1). However, due to several reasons, the ARR should be interpreted in the context of the plasma aldosterone concentration and plasma renin activity (PRA) or plasma direct renin concentration (DRC). Inappropriately low renin may lead to an artificially elevated ARR. The ARR also may be affected by any changes caused by physiological factors that can affect the renin-angiotensin-aldosterone system.
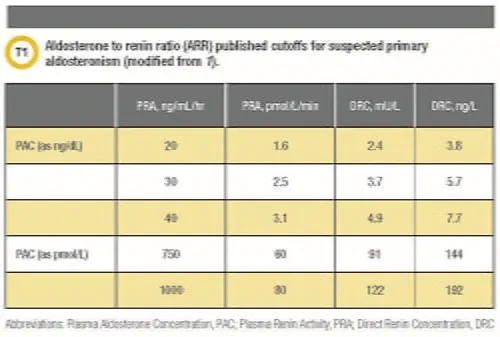
The Endocrine Society recommends several ARR cutoffs as a starting point for clinicians working up patients with suspected PA (Table 1). However, the methodology used to measure aldosterone and renin can have a huge impact on the interpretation of the ARR (3,4).
Measurement Methods for Aldosterone and Renin
Several different analytical methods are available to measure aldosterone (3). Gas chromatography mass spectrometry (MS) is considered the original reference method. Over time, commercially available and in-house-developed radioimmunoassays (RIA) became widespread but today are less favorable due to human and environmental radioactivity exposure concerns. Easy to use and automate, chemiluminescent immunoassays are also universally available, but they provide non-equivalent sensitivity and specificity compared with liquid chromatography tandem MS (LC-MS/MS) methods. LC-MS/MS is gaining support as the new reference method.
Laboratories can measure renin indirectly by PRA or directly as renin mass with DRC. PRA assays measure the generation of angiotensin I from angiotensinogen by renin’s enzymatic activity (3). Renin activity assays by LC-MS/MS are replacing older RIA methods (5).
DRC assays measure the enzyme’s direct concentration or enzyme’s presence. Although both approaches are used clinically in the diagnostic work-up of PA, each method offers inherent differences.
Renin assessed by PRA is a tried and true analytical methodology. The conversion of angiotensinogen to angiotensin I by renin needs to be performed in buffered conditions and often at two reaction temperatures (37°C and 4°C utilized as a “blank”). Reaction incubation times also can vary from 1 to 3 and even 18 hours depending on the concentration of renin in a patient’s sample (3).
Recent published methods of PRA by LC-MS/MS demonstrate improvements from the once popular RIA methods such as wide dynamic ranges (0.11-10.0 ng/mL/h), elimination of radiotracers and duplicate incubations, as well as the possibility of standardization of methods through the use of a high-quality calibrators available for angiotensin I (5). Even though PRA assays can be laborious, may contain radioisotopes as do RIA methods, or rely on expensive LC-MS/MS instrumentation and expertise, an abundance of literature demonstrates that they offer suitable low-end precision and clinically established cutoffs.
Assays that measure DRC utilize the basic capture and detection antibody schemes found in enzyme-linked immunosorbent assays or “sandwich assays” (3). These assays originally were immunoradiometric but since have been converted to automated chemiluminescent immunoassays. Unlike PRA, DRC assays are known to provide opportunities for walk-away automation, faster turnaround times, instrument connectivity to laboratory information systems, as well as a shared platform on which aldosterone can also be measured. However, limited supporting literature, poor correlations with PRA, and no clinically defined cutoffs limit widespread acceptance of this technique as a diagnostic test for PA. DRC assays are also susceptible to false increases due to conversion of renin to prorenin at temperatures around 4°C (3).
ARRs Present Interpretation Challenges
The ARR is considered a highly variable screening test that, if misinterpreted, can lead to delays in diagnosing and treating patients with PA (3). Diagnostic sensitivity of the ARR ranges from 64%-100% and specificity from 87%-100% (4). This high level of variability is attributed to within-subject variation, differences of laboratory assays used to measure renin or aldosterone, and many other preanalytical factors such as patient preparation protocols and prescribed medications. Additionally, ARR cutoffs provided in the 2016 Endocrine Society guidelines remain unchanged from the 2008 version even though advances have been made in aldosterone and renin methods such as LC-MS/MS and immunoassay (1,6).
The ARR may be falsely low or high due to several well-documented reasons (1). A good history and medication reconciliation are important to avoid testing patients with interfering factors that could lead to false-negative or false-positive results. False positives can be seen in hyperkalemic patients because this directly stimulates aldosterone production. Medications that directly inhibit renin can lower PRA while oral contraceptives or estrogens can directly lower DRC. On the other hand, hypokalemia impairs aldosterone production and can lead to false negatives. Mineralocorticoid receptor antagonist (MRA), angiotensin-converting enzyme inhibitors (ACE I), angiotensin II receptors (ARBs), diuretics, sodium restriction, and pregnancy all can cause false-negative ARR.
Specimen collection for aldosterone and renin testing should be performed mid-morning after a patient has been awake for at least 2 hours. Labs should avoid performing testing when samples have been obtained outside of appropriate collection times or in patients with interfering co-morbidities and/or medications. However, in some conditions it may not be feasible or safe to remove the confounding agent. Because of the importance of diagnosing patients with PA early, the Endocrine Society recommends that laboratories perform and interpret the ARR keeping these confounding factors in mind (1).
Diagnostic Teams Can Help Interpret ARRs
The challenges of a PA work-up demonstrate why laboratorians are critical to clinicians in diagnosing this disease. Recently the Institute of Medicine Committee on Diagnostic Error in Health Care endorsed using teams comprised of clinical experts to promote the diagnosis of many disorders (7). This report also identifies pathologists and other clinical laboratory experts as key contributors to these teams.
Such a group of experts is sometimes referred to as a diagnostic management team (DMT). A DMT is a collaborative group of laboratory and clinical experts who provide evidence-based, clinically useful, patient-specific interpretive reports that do not merely regurgitate laboratory data. To be considered DMTs, these groups must meet regularly (e.g. daily, weekly) to discuss cases and to formulate interpretations that utilize patient-specific clinical information that is valuable for patient care and readily viewable in electronic health records.
Patients being worked up for PA can benefit from the clinical expertise of a DMT that guides clinicians about ARR cutoffs for an institution’s assays and helps to identify confounders such as medications, abnormal potassium status during testing, and possible effects of renal insufficiency. All of this information should be considered in the context of a patient’s entire clinical picture to identify situations in which there is high likelihood of false-positive or false-negative results or identify results that are borderline and require additional work-up.
Implementing an endocrine DMT for suspected PA can positively impact patient care. Recently, Wiencek et al. performed a small pilot study to assess the effect of a DMT using patient-specific interpretive reports in facilitating appropriate diagnoses of patients being screened for PA (8). This retrospective cohort study included four primary care physicians (PCPs) and 32 patients with ARRs ordered before, and 27 patients after, a DMT was implemented. Before the DMT existed, four patients had unnecessary testing/procedures (e.g., diagnostic imaging and laboratory testing) and eight may have experienced diagnostic delays. After the DMT started, the four PCPs adhered closely to guidelines and DMT recommendations for all PA evaluations (e.g., appropriate repeat ARR, imaging, specialty consults, and/or confirmatory testing).
Provocative Confirmatory Testing
As mentioned, the ARR can be difficult to interpret because of the chance of false-positive and negative ratio results. For this reason, the Endocrine Society recommends provocative confirmatory testing (1). Currently, diagnostic work-ups of PA employ one or more of the following approaches:
- Oral sodium loading test. Patient is advised to increase sodium intake to about 6 grams (>200 mmol) for 3 days after which a 24-hour urine sodium and urine aldosterone is measured. A urinary aldosterone <10 μg/24 h (28 nmol/d) makes PA unlikely. An elevated urinary aldosterone >12 μg/24 h (>33 nmol/d) makes PA highly likely. This test is not recommended in patients with severe uncontrolled hypertension, renal insufficiency, cardiac arrhythmia, or severe hypokalemia.
- Saline infusion test. Patient stays in a recumbent position an hour before and during the infusion of 2 liters of 0.9% saline over 4 hours in the morning. Renin, aldosterone, cortisol, and potassium are drawn at time 0 and after 4 hours. BP and heart rate are monitored throughout the test. A post infusion aldosterone <5 ng/dL (140 pmol/L) makes PA less likely and levels >10 ng/dL (280 nmol/L) makes PA more likely. Values between 5-10 ng/dL are intermediate. This test is also contraindicated in patients with severe uncontrolled hypertension, renal insufficiency, cardiac arrhythmia, or severe hypokalemia.
- Fludrocortisone suppression test. Patient is given 0.1 mg of oral fludrocortisone every 6 hours for 4 days, potassium chloride supplements to keep plasma potassium close to 4.0 mmol/L, and high sodium diet to maintain urinary sodium excretion rate of at least 3 mmol/Kg body weight. On the fourth day, plasma aldosterone and PRA are measured at 10 a.m. and plasma cortisol at 7 a.m. and 10 a.m. Plasma aldosterone >6 ng/dL (170 nmol/L) on day 4 at 10 a.m. confirms PA provided PRA is <1 ng/mL/h and 10 a.m. plasma cortisol is lower than the 7 a.m. measurement.
- Captopril challenge test. Patient is given 25–50 mg of captopril orally after sitting or standing for at least 1 hour. PRA, plasma aldosterone, and cortisol are measured at time 0, and at 1 or 2 hours after the challenge, with the patient remaining seated during this period. Plasma aldosterone is normally suppressed by captopril but remains elevated while PRA remains suppressed in patients with PA. False negatives have been reported in patients with APA and in those with IAH.
There are certain instances in practice where confirmatory testing is not always performed. An example of this would be in a patient with spontaneous hypokalemia who has undetectable plasma renin with PAC >20 ng/dL (550 pmol/L). This is most likely PA and confirmatory testing is not necessary. Other patients might be unwilling or unable to proceed, in which case an MRA typically would be prescribed.
Subtype Classification for PA Treatment
All patients with confirmed PA should undergo subtype classification (1). Subtype classification is important because it helps to guide patient management. The recommended initial study is adrenal computed tomography (CT). A CT is vital to exclude large masses that may represent adrenocortical carcinoma and also to assist in making decisions for surgical interventions when appropriate.
Adrenal venous sampling (AVS) should be performed by an experienced radiologist before a patient proceeds to surgery. AVS is the gold standard test used in distinguishing between unilateral and bilateral adrenal disease (9). This test is operator-dependent and may be done unstimulated or Cosyntropin-stimulated under either bolus or continuous infusion conditions. Not all cases need to undergo AVS. Younger patients (age <35 years) with spontaneous hypokalemia, marked aldosterone excess, and unilateral adrenal lesions who have radiological features consistent with a cortical adenoma on adrenal CT scan may not need AVS before proceeding to unilateral adrenalectomy (9). The clinical and radiological findings are evident enough to be able to proceed with surgery.
The treatment of PA depends on subtype classification, surgical candidacy, and patient choice. Laparoscopic adrenalectomy is the treatment of choice for patients with unilateral PA. Patients do very well after surgery with normalization of plasma aldosterone and potassium concentrations. These patients end up with reduced or normalized blood pressure, requiring fewer to no antihypertensive medications. They also have reduced cardiac hypertrophy and fibrosis (9).
For patients unable or unwilling to undergo surgery, medical management with an MRA, such as spironolactone and eplerenone, are the treatment of choice. Notably, patients who have positive ARR but who are unwilling or unable to undergo further investigations may be treated medically with an MRA.
Medical management is recomÂmended for managing bilateral PA, such as in bilateral adrenal hyperplasia. Spironolactone is recommended as the primary agent, and eplerenone may be used as an alternative choice. Spironolactone is dosed once daily while eplerenone is dosed twice daily. Patients on medical management should have their potassium monitored as hyperkalemia is the most common side-effect of these medications.
Summary
PA remains a challenging diagnosis. Many factors influence the work-up of patients with suspected PA. As the overall rate of screening is expected to increase, collaboration between clinical laboratorians and other clinical experts will be key (1). Ultimately, a team-based approach could lead to helpful discussions about assay variability and diagnostic stewardship, which in turn could facilitate earlier disease detection.
Charity M. Kwamanakweenda, MD, is an endocrinology fellow in the Division of Endocrinology and Metabolism and Department of Medicine at University of Virginia School of Medicine. She starts a new position on September 1 at the University of Pittsburg in Pennsylvania. Email: [email protected]
Joesph R. Wiencek, PhD, is associate director of clinical chemistry in the Division of Laboratory Medicine and Department of Pathology at University of Virginia School of Medicine in Charlottesville. Email: [email protected]
References
- Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016;101:1889-916.
- Marcinkowski T. Conn’s syndrome or Lityn'ski-Conn syndrome? Mat Med Pol 1992;24:126-7.
- Rehan M, Raizman JE, Cavalier E, Don-Wauchope AC, Holmes DT. Laboratory challenges in primary aldosteronism screening and diagnosis. Clin Biochem 2015;48:377-87.
- O’Shea PM, Griffin TP, Browne GA, et al. Screening for primary aldosteronism using the newly developed IDS-iSYS automated assay system. Pract Lab Med 2016;7:6-14.
- Van Der Gugten JG, Holmes DT. Quantitation of Plasma Renin Activity in Plasma Using Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). Methods Mol Biol 2016;1378:243-53.
- Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008;93:3266-81.
- Committee on Diagnostic Error in Health Care; Board on Health Care Services; Institute of Medicine; The National Academies of Sciences, Engineering, and Medicine; Balogh EP, Miller BT, Ball JR, editors. Washington (DC): National Academies Press (US); 2015 Dec 29.
- Wiencek J, Colón-Franco J, Bissonnette S, Utz A, and Woodworth A. The endocrine diagnostic management team (DMT) pilot study. Am J Clin Pathol 2017;147:S170.
- Romero DG, Yanes Cardozo LL. Clinical Practice Guideline for Management of Primary Aldosteronism: What is New in the 2016 Update? Int J Endocrinol Metab Disord 2016;2:10.16966/2380-548X.129.
