Blood analysis is a cornerstone of diagnostic medicine, with serum and plasma serving as the primary matrices for a wide array of laboratory tests. Accurate clinical interpretation relies not only on the analytes being tested, but also on the appropriate specimen type and tube selection. BD Vacutainer® Blood Collection Tubes, specifically serum separator tubes (SST™), lithium heparin plasma separator tubes (PST™), and rapid clot serum tubes (RST™) are three tube types commonly used in routine clinical chemistry testing. Each tube type has unique characteristics that impact sample preparation, processing time, and test compatibility. Understanding the specific properties and appropriate use of these tubes can improve laboratory efficiency and patient care. This article provides a comprehensive comparison of these tubes, highlighting their differences, advantages, and limitations to support informed selection in clinical laboratory settings.
Serum is the clear, yellowish fluid portion of blood that remains after blood has clotted and the clot (including blood cells and clotting proteins like fibrinogen) has either been removed or separated via gel/barrier. It contains water, electrolytes, hormones, antibodies, antigens, and other proteins—except for the clotting factors. Serum is often used in routine clinical chemistry and serology testing.
Plasma, in contrast, is collected by centrifuging a blood tube containing an anticoagulant (e.g. heparin, ethylenediaminetetraacetic acid [EDTA], and citrate) thus preserving clotting factors within the liquid component. Citrated plasma is typically used in coagulation studies, such as prothrombin time (PT) and activated partial thromboplastin time (aPTT), and in certain biochemical tests where clotting proteins may be necessary or where sample stability is a concern.
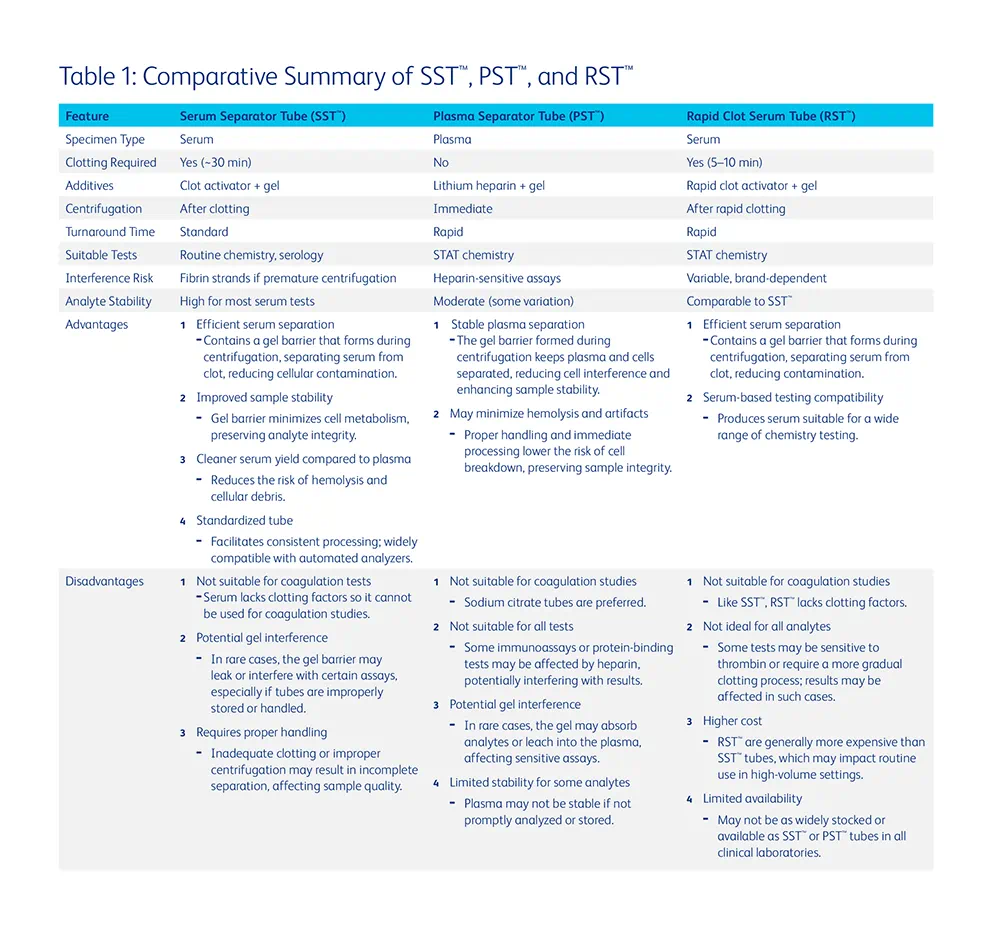
Modern clinical chemistry laboratories rely on specialized collection tubes to streamline the processing of serum and plasma samples. Three widely used tubes include: SST™, PST™, and RST™ (Table 1). SST™ are designed to collect serum after coagulation and centrifugation. They typically contain a clot activator and a gel barrier that separates serum from the clot during centrifugation. After collection, the sample must sit undisturbed for approximately 30 minutes to allow full clot formation prior to centrifugation. PST™ contain lithium heparin, an anticoagulant that prevents blood clotting, along with a gel separator. These tubes allow immediate centrifugation and separation of plasma, significantly reducing turnaround time. RST™ are designed to accelerate the clotting process, often using thrombin-based clot activators. These tubes typically allow clotting within 5 to 10 minutes, facilitating faster serum separation for urgent testing.
The distinctions between serum/plasma and the various tubes available must be carefully considered when deciding to implement a specific specimen or tube type in the laboratory. In general, tube selection should be guided by the test, urgency, and analytical method. Additionally, the impact on preanalytical variables should be considered when selecting a tube type. Serum is the most widely used specimen type for routine chemistry testing due to broad assay compatibility (1). However, there are several documented examples where one specimen or tube type confers a particular benefit over another.
Serum or SST™ specimens have been shown to offer better stability for some analytes when compared to plasma or PST™. For example, studies have demonstrated that the stability of glucose is greater in serum than in plasma samples stored at room temperature due to a lower degree of metabolism occurring in serum that is devoid of cells (1). Additional evidence highlights the risk of leucocytosis-induced plasma hyperkalemia upon pneumatic tube transport which can be mitigated through use of serum tubes (2).
The strongest arguments for the use of PST™ are made in the context of turnaround time and specimen yield. Plasma samples can be centrifuged immediately after collection. However, if a serum specimen is centrifuged before clot formation is complete (~30 min), residual fibrin or fibrinogen may interfere with instrument operation/testing and could lead to inaccurate results (3,4). Short turnaround time (TAT) is necessary to ensure appropriate therapeutic treatment in urgent patient care settings, specifically in the Emergency Department, operating rooms, and intensive care units. The use of PST™ can facilitate rapid result reporting in these time-sensitive scenarios. Plasma may also be the ideal choice when specimen volume is of concern. Centrifugation of anticoagulated specimens yields approximately 15-20% more volume compared to serum (1). This enables the use of low volume tubes which have been shown to reduce iatrogenic anemia in patient populations with reduced blood volumes (e.g. low birthweight infants) (1).
Recent publications have highlighted the efficacy of RST™, specifically in reducing preanalytical errors compared to traditional collection methods, while maintaining a short TAT (5–7) . A recent study conducted at Yale New Haven Hospital assessed the impact of tube type, fill volume, and collection methods on hemolysis and high-sensitivity cardiac troponin T (hs-cTnT) testing (5). The findings indicated that RST™ significantly reduced hemolysis compared to PST™. Proper tube filling further decreased hemolysis, underscoring the importance of correct collection techniques. Additionally, RST™ demonstrated a lower false positive rate for hs-cTnT levels <50 ng/L, suggesting improved assay accuracy in hemolyzed samples (5). A study comparing RST™ with traditional SST™ found that RST™provided shorter clotting times and were also less prone to hemolysis than SST™tubes (8). RST™ produced similar baseline results as SST™, consistent with other reports (9,10), making them a viable alternative for reducing processing time while maintaining sample quality.
To ensure optimal sample quality and test accuracy, consider the following best practices:
- Match Tube Type to Test Requirements: Choose the tube type based on the specific requirements of the test detailed in the manufacturer’s instructions for use, such as the need for serum or plasma or sensitivity to additives like heparin.
- Validate any tubes that will be used clinically: Conduct a study evaluating analyte comparability, assessing possible bias according to other method comparisons outlined in CLSI EP09c (11), as required by regulatory bodies such as the College of American Pathologists.
- Assess reference interval comparability: Verify reference intervals with all specimen types as specimen source-specific reference intervals may be necessary for analytes like potassium and total protein.
- Match tube to urgency: Use PST™ or RST™ for STAT analyses; SST™ for routine testing.
- Ensure proper handling: Adhere to manufacturer protocols for mixing, clotting, and centrifugation.
- Train personnel: Staff should be trained to recognize the differences in tube types and the impact of improper handling.
The selection of the appropriate blood collection tube is crucial in clinical chemistry laboratories to ensure timely, accurate, and reliable test results. By understanding the characteristics, applications, and limitations of SST™, PST™, and RST™, laboratory professionals can make informed decisions that optimize patient care and laboratory efficiency. Optimal use depends on balancing speed, assay compatibility, and analyte stability. Adhering to best practices in tube selection and handling can significantly reduce preanalytical errors, improve the overall quality of laboratory diagnostics, and enhance patient outcomes.
References
- Plebani M, Banfi G, Bernardini S, Bondanini F, Conti L, Dorizzi, et al. Serum or plasma? An old question looking for new answers. Clin Chem Lab Med [Internet]. De Gruyter Open Ltd; 2020 [cited 2025 May 22];58:178–87
- Grzych G, Pekar JD, Maboudou P, Lippi G. Leucocytosis-induced plasma hyperkalaemia in samples conveyed by a pneumatic transport system: tips and tricks [Internet]. Br. J. Haematol. John Wiley & Sons, Ltd; 2019 [cited 2025 May 22]. page e71–3. Available from: /doi/pdf/10.1111/bjh.15908
- Nosanchuk JS, Combs B, Abbott G. False increases of troponin I attributable to incomplete separation of serum [Internet]. Clin. Chem. Oxford Academic; 1999 [cited 2025 May 22]. page 714. Available from: https://dx.doi.org/10.1093/clinchem/45.5.714
- Roberts WL, Calcote CB, De BK, Holmstrom V, Narlock C, Apple FS, et al. Prevention of analytical false-positive increases of cardiac troponin I on the Stratus II analyzer [Internet]. Clin. Chem. Oxford Academic; 1997 [cited 2025 May 22]. page 860–1. Available from: https://dx.doi.org/10.1093/clinchem/43.5.860
- Malaeb H, Vera MA, Sangal RB, Venkatesh AK, Possick S, Maciejak L, et al. Rapid serum tubes reduce transport hemolysis and false positive rates for high-sensitivity troponin T. Clin Chim Acta [Internet]. Elsevier B.V.; 2023 [cited 2025 May 22];551:117630. Available from: https://pubmed.ncbi.nlm.nih.gov/38420909/
- Koch CD, Wockenfus AM, Saenger AK, Jaffe AS, Karon BS. BD rapid serum tubes reduce false positive plasma troponin T results on the Roche Cobas e411 analyzer. Clin Biochem. 2012;45:842–4.
- Ryan J, Stuart L, Southby S, Than M, Mackay R, Florkowski C, et al. Comparison of BD Vacutainer® Rapid Serum Tube and plasma for haemolysis markers in the emergency department. Ann Clin Biochem. SAGE Publications Ltd; 2015;52:293–6.
- Ayala-Lopez N, Conklin SE, Tenney BJ, Ness M, Marzinke MA. Comparative evaluation of blood collection tubes for clinical chemistry analysis. Clin Chim Acta [Internet]. Elsevier; 2021 [cited 2025 May 22];520:118–25. Available from: https://www.sciencedirect.com/science/article/pii/S0009898121001753?via%3Dihub
- Yan R, Lou A, Watts G, Tarr H, Smith H, Kinney L, et al. Comparison of Becton Dickinson Vacutainer rapid serum tube with the serum separator tube for routine chemistry and immunoassay tests. J Clin Pathol [Internet]. BMJ Publishing Group; 2014 [cited 2025 May 22];67:599–604. Available from: https://jcp.bmj.com/content/67/7/599
- Seo J Do, Lee YJ, Ha C, Choi JH, Shin SH, Han HY, et al. Comparison of three blood collection tubes for 16 biochemical analytes and stability assessment of selected analytes: VACUETTE® CAT serum Sep clot activator tube, VACUETTE® LH lithium heparin Sep tube, and VACUETTE® CAT serum fast separator tube. Ann Clin Biochem [Internet]. SAGE Publications Ltd; 2025 [cited 2025 May 22]; Available from: https://journals.sagepub.com/doi/10.1177/00045632251329272
- CLSI. Measurement Procedure Comparison and Bias Estimation Using Patient Samples. 3rd ed. CLSI guideline EP09c. Wayne, PA; Clinical and Laboratory Standards Institute; 2018.